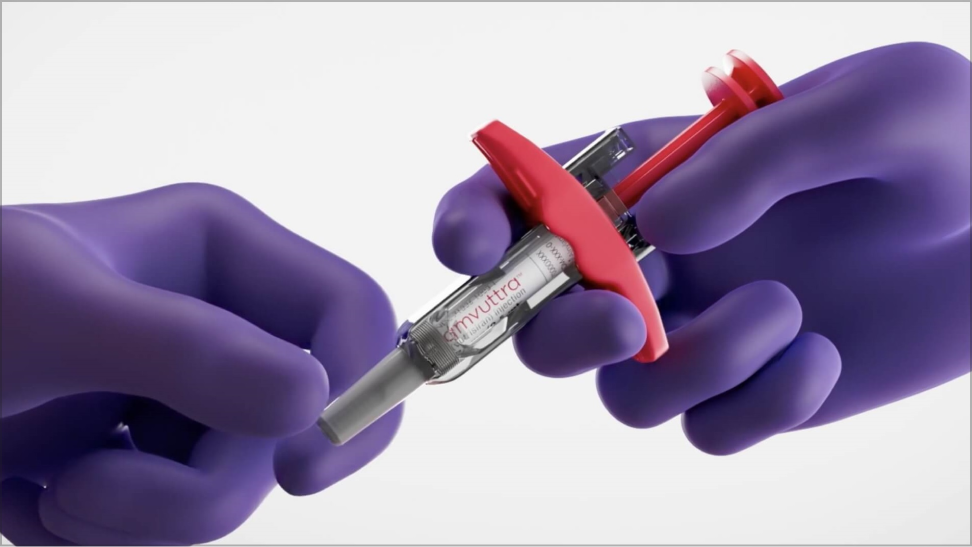
Dosing & Administration
AMVUTTRA® delivers clinical benefits with only 4 doses per year1
Administering AMVUTTRA
- The recommended dose of AMVUTTRA is 25 mg administered by subcutaneous injection once every 3 months by a healthcare professional1
- AMVUTTRA is provided as a fixed dose (25 mg/0.5 mL) prefilled syringe—no dose adjustments required1
- Injection site reactions may occur1
(See section 2.2 of full Prescribing Information for complete Administration Instructions.)
Missing a dose
- If a dose is missed, administer AMVUTTRA as soon as possible. Resume dosing every 3 months from the most recently administered dose1
AMVUTTRA requires the fewest doses to treat ATTR-CM and hATTR-PN1,3-6*
*No head-to-head trials have been conducted. Conclusions about similarities and/or differences in clinical profiles cannot be drawn.
ATTR-CM=cardiomyopathy of transthyretin-mediated amyloidosis; hATTR-PN=polyneuropathy of hereditary transthyretin-mediated amyloidosis; HCP=healthcare professional.
To learn more about dosing and administration:
AMVUTTRA offers confidence in monitoring patient compliance with
HCP-administered doses only four times per year1