HELIOS-B Efficacy
AMVUTTRA® significantly reduced the risk of ACM and recurrent CV events1
Primary composite endpoint analysis through 33-36 months1
Relative risk reduction
of ACM and recurrent CV events
(N=654)
[HR=0.72 (95% CI: 0.55–0.93); p=0.01]
Relative risk reduction
of ACM and recurrent CV events
(N=395)
[HR=0.67 (95% CI: 0.49–0.93); p=0.02]
4 patients in the overall population and 3 patients in the monotherapy population need to be treated to prevent 1 death or CV event in the DB period2*

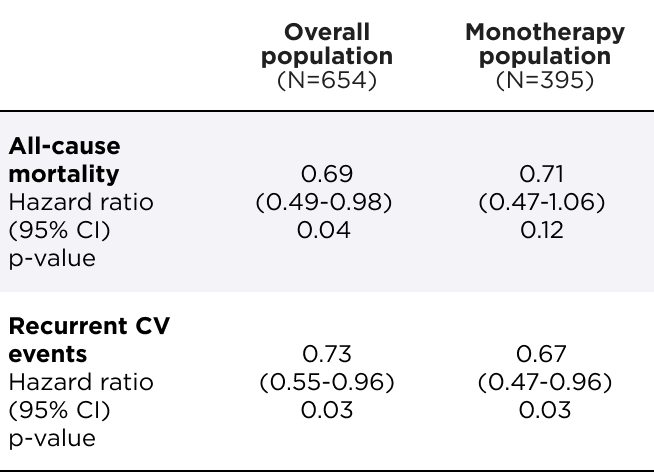
*During the 33-36 month DB period, NNT=3.4 in the overall population and 2.7 in the monotherapy population. ARR (per 100 patient-years) was 9.9 in the overall population and 12.5 in the monotherapy population.2
ARR=absolute risk reduction; DB=double-blind; NNT=number needed to treat.
Time to First CV Event or All-Cause Mortality (Overall Population)1*†‡


*The Kaplan-Meier curves are unadjusted for baseline imbalances in disease-severity characteristics.3
†Heart transplantation and left ventricular assist device placement are treated as death. HR and 95% CI are based on a Cox proportional-hazards model.1
‡Data were censored at each patient's first dose in the OLE, which could occur at approximately 33 or 36 months, depending on the patient's enrollment time. For patients who did not enter the OLE, data were censored at their study discontinuation date.3
CI=confidence interval; CV=cardiovascular; DB=double-blind; HR=hazard ratio; OLE=open-label extension.
AMVUTTRA achieved consistent results across all prespecified subgroups1

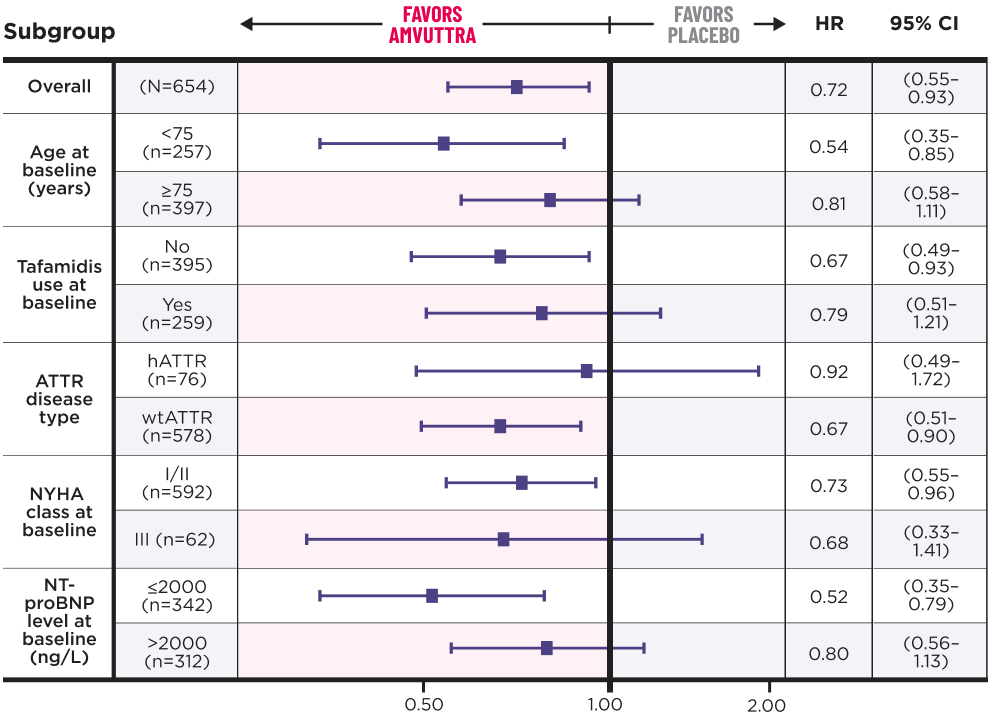
At the end of the DB period, all remaining patients on placebo transitioned to AMVUTTRA treatment in the OLE.3
An updated analysis was conducted to incorporate additional patient follow-up data, which was not controlled for multiplicity.4§
All-Cause Mortality (Overall Population)2,4,5*†‡


*The Kaplan-Meier curves are unadjusted for baseline imbalances in disease-severity characteristics.3
†Heart transplantation and left ventricular assist device placement are treated as death.6
‡For patients in both treatment arms, survival time was censored 6 months after the first dose in the OLE period.3
§Initial analysis (May 2024 data cut): 42.4% of patients with data through 42 months. Updated analysis (Nov 2024 data cut): 96.3% of patients with data through 42 months.4
CI=confidence interval; HR=hazard ratio.
Significant benefit observed in functional capacity and quality of life for patients treated with AMVUTTRA compared with placebo1,6


- Healthy adults without ATTR-CM experience a natural decline in 6‑MWT of 5‑6 meters per year7
- A 5-point change in KCCQ‑OS (a measure of health status and health‑related QoL) is considered clinically meaningful8
*6-MWT assesses functional capacity by measuring distance walked over a period of 6 minutes. A decrease in the distance walked indicates a decline in functional capacity.9
†The KCCQ is a 23-item, self-administered questionnaire that measures the patient’s perception of health status within a 2-week recall period (scoring on KCCQ-OS: 0 to 100, with a higher score indicating better quality of life).3
6-MWT=6-minute walk test; CI=confidence interval; KCCQ-OS=Kansas City Cardiomyopathy Questionnaire-Overall Summary; LS=least squares; SE=standard error.
Patients receiving AMVUTTRA maintained relative stability in functional capacity and quality of life3




QoL=quality of life.
Biomarkers associated with heart failure favored AMVUTTRA over placebo1


- Analyses were exploratory and not adjusted for multiplicity.3









